Docosahexaenoic acid (DHA) influences body’s immune function and inflammation; however, the influence of maternal DHA supplementation on incidence of diseases in infants is unknown. A double-blind, randomized, controlled trial was conducted to evaluate the effects of prenatal DHA supplementation on infant morbidity.
Pregnant women
From 18 to 22 weeks' of pregnancy until childbirth
400 mg of DHA supplementation or placebo
In infants of age 1, 3 and 6 months, caregivers reported the occurrence of common illness symptoms in the preceding 15 days
Data were available at 1, 3 and 6 months for 849, 834, and 834 infants, respectively.
The DHA group experienced 26%, 15% and 30% shorter duration of cough, phlegm, and wheezing, respectively at one-month age.
At 3 months, infants in the DHA group spent 14% less time being sick (p<0.0001).
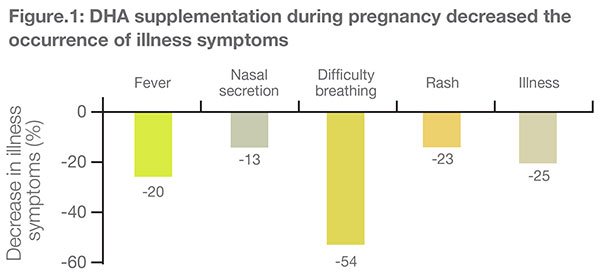
At 6 months, infants in the DHA group experienced 20%, 13%, 54%, 23% and 25% shorter duration of fever, nasal secretion, difficulty in breathing, rash and "other illness," respectively (Figure 1).
DHA supplementation during pregnancy decreased the occurrence of colds in 1-month-old children and influenced duration of disease symptoms in 1, 3 and 6 months old children.
Imhoff-Kunsch B, Stein AD, Martorell R et al. Prenatal docosahexaenoic acid supplementation and infant morbidity: randomized controlled trial. Pediatrics. 2011;128(3):e505-e512.